Organisms are composed of matter, which is anything that takes up space and has mass.· Matter exists in many diverse forms. Rocks, metals, oils, gases, and humans are just a few examples of what seems an endless assortment of matter.
Elements and Compounds
Matter is made up of elements. An clement is a substance that cannot be broken down to other substances by chemical reactions. Today, chemists recognize 92 elements occurring in nature; gold, copper, carbon, and oxygen are examples. Each element has a symbol, usually the first letter or two of its name. Some symbols are derived from Latin or German; for instance, the symbol for sodium is Na, from the Latin word natrium.
Matter is made up of elements. An clement is a substance that cannot be broken down to other substances by chemical reactions. Today, chemists recognize 92 elements occurring in nature; gold, copper, carbon, and oxygen are examples. Each element has a symbol, usually the first letter or two of its name. Some symbols are derived from Latin or German; for instance, the symbol for sodium is Na, from the Latin word natrium.
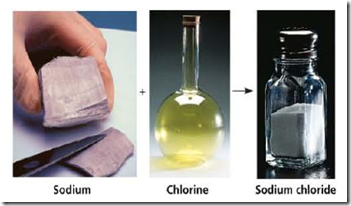
A compound is a substance consisting of two or more different elements combined in a fixed ratio. Table salt, for example, is sodium chloride (NaG), a compound composed of the elements sodium (Na) and chlorine (el) in a 1:1 ratio. Pure sodium is a metal, and pure chlorine is a poisonous gas. When chemically combined, however, sodium and chlorine form an edible compound. Water (H 2 0), another compound, consists of the elements hydrogen (H) and oxygen (0) in a 2:1 ratio. These are simple examples of organized matter having emergent properties: A compound has characteristics different from those of its elements. See these figures below!
The emergent properties of a compound. The metal sodium combines with the poisonous gas chlorine, forming the edible compound sodium chloride, or table salt.
*Sometimes we substitute the term weight for mass, although the two are not identical. Mass is the amount of matter in an object. whereas the weight of an object is how strongly that mass is pulled by gravity. The weight of an astronaut walking on the moon is approximately II that of the astronauts weight on Earth, but his or her mass is the same.
Essential Elements of Life About 25 of the 92 natural elements are known to be essential to life. Just four of these-carbon (C), oxygen (0), hydrogen (H), and nitrogen (N)-make up 96% of living matter. Phosphorus (Pl, sulfur (S), calcium (Ca), potassium (K), and a few other elements account for most of the remaining 4% of an organism's weight. Table 2.1 lists by percentage the elements that make up the human body; the percentages for other organisms are similar. Figure 2.4a illustrates the effect of a deficiency of nitrogen, an essential element, in a plant.
Trace elements are those required by an organism in only minute quantities. Some trace elements, such as iron (Fe), are needed by all forms of life; others are required only by certain species. For example, in vertebrates (animals with backbones), the element iodine (I) is an essential ingredient of a hormone produced by the thyroid gland. A daily intake of only 0.15 milligram (mg) of iodine is adequate for normal activity of the human thyroid. An iodine deficiency in the diet causes the thyroid gland to grow to abnormal size, a condition called goiter. Where it is available, iodized salt has reduced the incidence of goiter.
An Element's properties depend on the structure of its atoms. Although the atom is the smallest unit having the properties of its element, these tiny bits of matter are composed of even smaller parts, called subatomic particles. Physicists have split the atom into more than a hundred types of particles, but only
Simplified models of a helium (He) atom. The helium nucleus consists of 2 neutrons (brown) and 2 protons (pink), Two electrons (yellow) exist outside the nucleus, These models are not to scale; they greatly overestimate the size of the nucleus in relation to
the electron cloud.
the electron cloud.
Three kinds of particles are relevant here: neutrons, protons, and electrons. Protons and electrons are electrically charged. Each proton has one unit of positive charge, and each electron has one unit of negative charge. A neutron, as its name implies, is electrically neutral.
Protons and neutrons are packed together tightly in a dense core, or atomic nucleus, at the center of an atom; protons give the nucleus a positive charge. The electrons form a sort of cloud of negative charge around the nucleus, and it is the attraction between opposite charges that keeps the electrons in the vicinity of the nucleus. Figure above shows two models of the structure of the helium atom as an example.
The neutron and proton are almost identical in mass, each about 1.7 X 10- 14 gram (g). Grams and other conventional units are not very useful for describing the mass of objects so minuscule. Thus, for atoms and subatomic particles (and for molecules, too), we use a unit of measurement called the dalton, in honor of John Dalton, the British scientist who helped develop atomic theory around 18()(). (The dalton is the same as the atomic mass unit, a unit you may have encountered elsewhere.) Neutrons and protons have masses close to 1 dalton. Because the mass of an electron is only about 1/2000 that of a neutron or proton, we can ignore electrons when computing the total mass of an atom.
Atomic Number and Atomic Mass
The image above is a simplified models of a helium (He) atom. The helium nucleus consists of 2 neutrons (brown) and 2 protons (pink), Two electrons (yellow) exist outside the nucleus, These models are not to scale; they greatly overestimate the size of the nucleus in relation to the electron cloud.
Atoms of the various elements differ in their number of subatomic particles. All atoms of a particular element have the same number of protons in their nuclei. This number of protons, which is unique to that element, is called the atomic number and is written as a subscript to the left of the symbol.
Atoms of the various elements differ in their number of subatomic particles. All atoms of a particular element have the same number of protons in their nuclei. This number of protons, which is unique to that element, is called the atomic number and is written as a subscript to the left of the symbol All atoms of a given element have the same number of protons, but some atoms have more neutrons than other atoms of the same element and therefore have greater mass. These different atomic forms are called isotopes of the element.
In nature, an element occurs as a mixture of its isotopes. For example, consider the three isotopes of the element carbon, which has the atomic number 6. The most common isotope is carbon-l2, IJc, which accounts for about 99% of the carbon in nature. The isotope IJc has 6 neutrons. Most of the remaining 1% of carbon consists of atoms of the isotope lJC, with 7 neutrons. A third, even rarer isotope, has 8 neutrons.
Notice that all three isotopes of carbon have 6 protons; otherwise, they would not be carbon. Although the isotopes of an element have slightly different masses, they behave identically in chemical reactions. (The number usually given as the atomic mass of an element, such as 22.9898 daltons for sodium, is actually an average of the atomic masses of all the element's naturally occurring isotopes.)
Both 11C and 13C are stable isotopes, meaning that their nuclei do not have a tendency to lose particles. The isotope He, however, is unstable, or radioactive. A radioactive isotope is one in which the nucleus decays spontaneously, giving off particles and energy. When the decay leads to a change in the for the element. The abbreviation 1He, for example, tells us that an atom of the element helium has 2 protons in its nucleus.
Unless otherwise indicated, an atom is neutral in electrical charge, which means that its protons must be balanced by an equal number of electrons. Therefore, the atomic number tells us the number of protons and also the number of electrons in an electrically neutral atom.
We can deduce the number of neutrons from a second quantity, the mass number, which is the sum of protons plus neutrons in the nucleus of an atom. The mass number is written as a superscript to the left of an element's symbol. For example, we can use this shorthand to write an atom of helium as He. Because the atomic number indicates how many protons there are, we can determine the number of neutrons by subtracting the atomic number from the mass number: The
helium atom, He, has 2 neutrons. An atom of sodium, Na, has 11 protons, 11 electrons, and 12 neutrons.
helium atom, He, has 2 neutrons. An atom of sodium, Na, has 11 protons, 11 electrons, and 12 neutrons.
The simplest atom is hydrogen, H, which has no neutrons; it consists of a single proton with a single electron. As mentioned earlier, the contribution of electrons to mass is negligible. Therefore, almost all of an atom's mass is concentrated in its nucleus. Because neutrons and protons each have a mass very close to 1 dalton, the mass number is an approximation of the total mass of an atom, called its atomic mass. So we might say that the atomic mass of sodium (Na) is 23 daltons, although more precisely it is 22.9898 daltons.


Komentar
Posting Komentar
Silahkan memberi komentar yang baik dan membangun. Sampaikan saran, kritik, pertanyaan, atau opini Anda. Kami akan coba lakukan yang terbaik untuk sobat Zona Biologi Kita